 Hi, readers welcome to the new post. in this post, we will have a detailed look at Difference Between Heat and Temperature. both of these are somewhat different but have some common relation to one another. Heat is type of energy and temperature discusses the quantity of thermal energy. The common relation among them is that with the larger value of heat there will rise in temperature.
Hi, readers welcome to the new post. in this post, we will have a detailed look at Difference Between Heat and Temperature. both of these are somewhat different but have some common relation to one another. Heat is type of energy and temperature discusses the quantity of thermal energy. The common relation among them is that with the larger value of heat there will rise in temperature.
With the decrement in heat temperature decreases Here we discuss the parameters of these two with detail. So let get started Difference Between Heat and Temperature.
Difference Between Heat and Temperature
Introduction to Heat
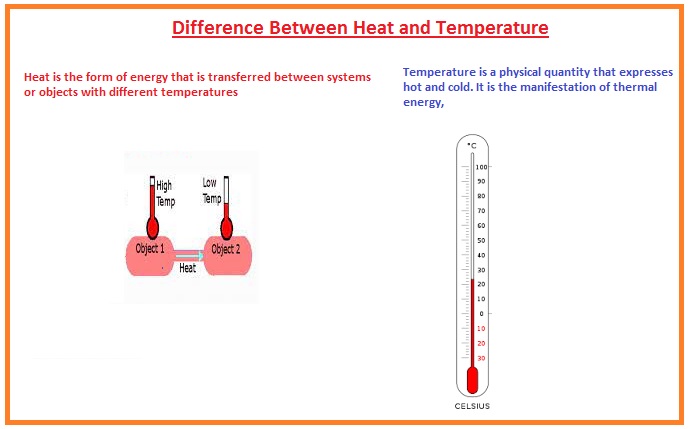
- Heat is a type of energy that is transmitted among the different bodies having different values of temperature
- It also named thermal energy. its measuring units are British thermal units or joule.
How Heat Transfer
- There are 3 main types of heat transfer processes that are commonly observed in our environment.
- The first one is through radiation second one conductin and the third one is convection.
- The process through which the transfer of heat occurs through a physical connection between two objects called conduction.
- The process of heat transfer in which heat transferred through the motion of objects like electrons moves that heat transferred or generated.
- The phenomenon through which heat transfer from electromagnetic waves called radiation
What is Temperature
- temperature is a term that explains the degree of coldness and hotness of any object,
- the thermometer is used to measure temperature. its measuring units are centigrade Kelvin and Fahrenheit.
- the least value of temperature called absolute, It is such a temperature where there is no heat removed from the object.
- Galileo first time noted the temperate in history. He used an instrument known as a thermocouple for mearing fo temperature.
Temperature Scales
- Generally, three types of temperature scales used but centigrade is most commonly use. Its minimum point is zero that is the water freezing point and the highest points is one hundred that is the water boiling point
- The other two units used are Kelvin and Fahrenheit.
What is Absolute zero
- the temperature over which zero value of heat get through the object called absolute zero. At this temperature, there is no motion occurred in the particles of body.
- Its value is zero kelvin and in centigrade −273.15 and Farhenheirt −459.67
That is a detailed post about Difference Between Heat and Temperature if you has any further query ask in the comment. Thanks for reading have a nice day.