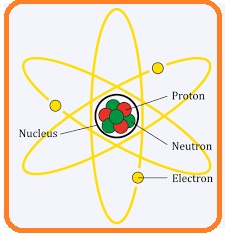
 Hello, friends welcome to the new post. in this post, we will have a detailed look at Difference Between Electron and Proton. The atom is the least portion of any substance that used to create an object. There are further three main sub-parts of the atoms first one is an electron second one is a proton and the third one is a neutron. Atoms is considered as the main element of any substance since it is created through the connection of different atoms. Atom has positive charge when its release electrons and has a negative charge when getting the electrons
Hello, friends welcome to the new post. in this post, we will have a detailed look at Difference Between Electron and Proton. The atom is the least portion of any substance that used to create an object. There are further three main sub-parts of the atoms first one is an electron second one is a proton and the third one is a neutron. Atoms is considered as the main element of any substance since it is created through the connection of different atoms. Atom has positive charge when its release electrons and has a negative charge when getting the electrons
There is a large number of atoms used to create molecules and these atoms are combine through chemical bonding. If we do compassion between electron and proton then come to know that there is positive charge at proton and negative at electrons. Both of these components are fundamental parts of atoms. So let’s get started.
Difference Between Electron and Proton
Introduction to Electrons
- The smallest part of atoms of any material that is denoted with the letter e and has a charge of negative value.
- It is part of a lepton group of different particles and it’s the main component of the structure of ant material.
- Its mass is 1/1836 time of protons mass that is also part of the atom and has a positive charge.
- It shows both behaviors like wave and particles when strike with other objects shows diffraction behavior like light.
- Its wave feature can be detected through different experiments.
- This article helps to sturdy the different educations lie electricity, chemistry, physics, etc.
- Due to the charge on the electron, there is an electric field exist about it and moving electron shows a magnetic field.

what is Protons
- The proton is also a particle of atoms that exists in the inner side of atoms in the nucleus and has a positive charge.
- Its mass is less than the neutron that exists with it in the nucleus.
- Singel or large protons exist in the nueleus The number of protons existing in the atoms tells about the atomic number that is denoted with the letter Z.
- Every element have a different value of Z due to different number of protons
- The atoms that have the same number of proton and neutron that atoms has zero charge
- the ahrge at protons is 1.6021 x 10^-19
- Its removal and addition in the atoms is the difficult to process like the electron.
that is a detailed post about Difference Between Electron and Proton if you have any further query ask in a comment, Thanks for reading. have a nice day.






