Hello guys welcome to the new tutorial. In this post, we will discuss Different Types of Battery. A battery is an electrical device that is used to give power to the system and store electrical energy in form of chemical energy. The battery is created with a combination of cells that stored the energy in form of chemicals. It is also named electrochemical modules. There are different devices that consist of batteries to work on such as laptops. Mobiles. watches lights, etc.
Hello guys welcome to the new tutorial. In this post, we will discuss Different Types of Battery. A battery is an electrical device that is used to give power to the system and store electrical energy in form of chemical energy. The battery is created with a combination of cells that stored the energy in form of chemicals. It is also named electrochemical modules. There are different devices that consist of batteries to work on such as laptops. Mobiles. watches lights, etc.
Different types of batteries are used at different levels according to design and construction. Here we discuss all types of batteries with the details and their applications. So let’s get started.
Types of Battery
- There are two main types of batteries according to charging and not charging combination
- Primary that can not be charged
- Secondary Battery Rechargeable
- These are 2 main types but there are further subtypes we discuss all these in detail.
Primary Battery
- It is such a category of battery that is used for one time and after that become useless mean cannot charge again. It is also called a voltaic battery.
- As it has disadvantages that can not recharge but has some features like less cost and easy to use..
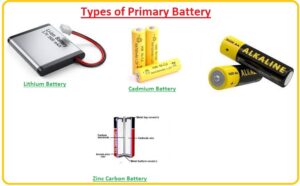
Types of Primary Battery
- Different types of primary batteries are described here with the details.
Lithium Battery
- This category of battery is very commonly employed in different projects since there is different innovation made in it. These are preferred to use since can handle different temperature values and operates for long time interval.
Cadmium Battery
- It is designed with the use of mercury and cadmium and is mostly used for fewer applications since cadmium has toxic behavior but is also used in place of zinc batteries.
Alkaline battery
- It is also called zinc and manganese dioxide battery. It is somewhat is costly and mostly used.
Zinc Carbon Battery
- It is also called leclanche cell or carbon cell battery and very commonly used. Its common features are high reliability, less cost.
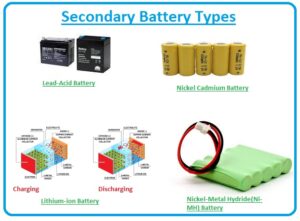
Secondary Battery Types
- The main types of secondary batteries are described here with detail.
- Lead-Acid
- Lithium-ion(Li-ion)
- Nickel Cadmium
- Nickel-Metal Hydride(Ni-MH)
Lead-Acid Battery
- It can be recharged after discharging process and the first time was introduced by Gaston Plante in 1859.
- WIth The interesting thing is that it is the first rechargeable battery that was used and invented.
- As compared to other batteries there is it uses less energy density. But it offered a larger value of power-to-weight ratio.
- Since its price is less soused for starting of motor.
- When it is charged stored the energy in form of potential energy at the electors created with the lead that is cathode and PbO2 is positive or anode.
Lithium-ion Battery
- It advanced types of battery that ues lithium ions the main elements of battery.
- When this battery is discharged there ionization process occurs at the anode for lithium atoms and losses the electrons.
- These ions go to the cathode and combine with the electrons and become neutral.
- This battery offers a large value of volts and stores a larger amount of charges.
- It is normally used in mobiles watches and instruments used for military purposes.
Nickel Cadmium Battery
- It is a rechargeable battery and consists of electrons of nickel oxide hydroxide and cadmium.
- It was designed in 1899 and its electrodes volts value is 1.2 volts and decreases to zero through discharge.
- The extreme emf force given through this cell is 1.3 volts. It exists in different ranges and sizes of ratings.
- Their operation life is good also works at less value of temperature and offers a high discharge completely rated value
- It was very commonly used in power devices, photography devices, lights, and some other.
Nickel-Metal Hydride(Ni-MH) Battery
- It is a rechargeable battery the reaction done at the electrodes of this module is like the NiCd battery.
- There are electrodes of hydrogens are used in place of cadmium. It is operation range is larger than the same range of NiCd battery.
- Mostly these batteries are used in place of alkaline batteries since has compatibility with volts
20 main types of battery and features
| Battery Type | Features |
|---|---|
| Alkaline | Long-lasting, non-rechargeable, and frequently used in low-drain devices |
| Zinc-Carbon | Low-cost, non-rechargeable, and frequently utilized in low-drain devices |
| Lithium | High power output, extended shelf life, non-rechargeable, widely used in watches and digital cameras |
| Mercury | Stable voltage, non-rechargeable, and being phased out because of environmental issues |
| Lead-Acid | Rechargeable, frequently seen in automobiles, large and hefty |
| Nickel-Cadmium | High energy density, rechargeable, and being phased out because of environmental issues |
| Nickel-Metal Hydride | High energy density, rechargeable batteries are frequently employed in portable gadgets. |
| Lithium-Ion | High energy density, rechargeable batteries are frequently found in computers and cell phones. |
| Lithium-Polymer | often seen in wearable technology, rechargeable batteries are flexible and light. |
| Nickel-Iron | Long lifetime, frequently utilized in renewable energy systems, and rechargeable |
| Silver-Zinc | High energy density, rechargeable, and frequently seen in hearing aids |
| Silver-Cadmium | High energy density, rechargeable, and being phased out because of environmental issues |
| Zinc-Air | High energy density, non-rechargeable, and frequently seen in hearing aids |
| Sodium-Sulfur | Utilized frequently in grid-scale energy storage, rechargeable, and high energy density |
| Flow | Scalable, rechargeable, and frequently utilized for grid-scale energy storage |
| Solid State | Rechargeable, durable, secure, and often used in electric cars |
| Sodium-Nickel Chloride | Frequently used in electric cars, rechargeable, and high in energy density |
| Lithium-Sulfur | High energy density, rechargeable batteries are being developed for electric cars. |
| Aluminum-Air | High energy density, non-rechargeable batteries are being created for electric cars. |
| Redox | Long-lasting, rechargeable, and being created for grid-scale energy storage |
That is all about the Types of Battery. I have explained all types of batteries with the details if you still have any further query ask in the comments. Thanks for reading have a nice day.