 Hello guys, I hope you all are doing great. In today’s tutorial, we will discuss the Difference Between Valence Band and Conduction Band. Both valence and conduction bands are energy levels which has some amount of energy difference. the valence band denotes the energy level of electrons existing in the outermost shell or valence shell of an atom.
Hello guys, I hope you all are doing great. In today’s tutorial, we will discuss the Difference Between Valence Band and Conduction Band. Both valence and conduction bands are energy levels which has some amount of energy difference. the valence band denotes the energy level of electrons existing in the outermost shell or valence shell of an atom.
While in conduction band such electrons exist that take part in the process of conduction. In today’s post, we will have a detailed look at both valence and conduction band to find their different parameter and compare them to discuss their differences. So let’s get started with Difference Between Valence Band and Conduction Band.
Difference Between Valence Band and Conduction Band
Valance Band
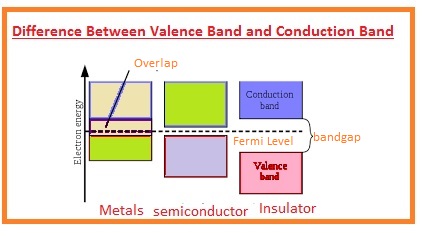
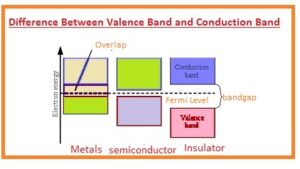
- The valence band is denoted with the VB.
- The location of this band is below the Fermi level.
- It is an electron orbital from which electrons can move to the condition band after getting excitation.
- The electrons existing In the most outer shell called the valence band.
- The energy gap between the valence gap and conduction gap called a bandgap.
- The existence of valence electrons explains the chemical feature of any component.
- For the main group, the valence electrons rely on in the outer shell of the electron, and in transition metal exist in the internal shell
- Due to the exterior excitation process electron left the valance band.
- Due to the existence of electrons valance band is partially or completely filled.
- The energy state of this band is less.
- In this band, there are a large number of electrons present.
- The force exerted on this band due to the nucleus is stronger.
Conduction Band
- This band is orbited in the atom where the electrons go after excitation from the valance band.
- In orbits, electrons can move freely and cause the flow of current
- The conduction band is denoted with the CB.
- Conduction band present over the Fermi level.
- Due to exterior excitation electrons enter the conduction band.
- Its energy state is larger than the valance band.
- The concentration of electrons in this band is less.
- The force exerted on the valance band due to the nucleus is less strong.
- The conduction is not filled or partially filled
That is a detailed post about the difference between conduction band and valence band. If you have any query ask in comments. Thanks for reading. have a good day.