 Hello fellows, I hope you all are doing great. In today’s tutorial, we will have a look at Difference between Ionic and Covalent Bond. In atoms, there are two bonds that exist first one is a covalent bond and the second one is an ionic bond. These bonds have different features and structures. A covalent bond is created with the pairing of electrons of 2 atoms and this pairing combines the atom in a certain arrangement. To breaking the covalent bond large amount of energy is usually needs fifty to two hundred Kcal/mol.
Hello fellows, I hope you all are doing great. In today’s tutorial, we will have a look at Difference between Ionic and Covalent Bond. In atoms, there are two bonds that exist first one is a covalent bond and the second one is an ionic bond. These bonds have different features and structures. A covalent bond is created with the pairing of electrons of 2 atoms and this pairing combines the atom in a certain arrangement. To breaking the covalent bond large amount of energy is usually needs fifty to two hundred Kcal/mol.
2 atoms create covalent bonds according to their electronegativity which is the energy of the atom in a molecule to have a force of attraction for electrons. It 2 atoms have different electronegativities like atoms of sodium and chlorine then one atom will give an electron to another atom. It makes positive ion and negative ion, positive ions make for atom which releases electrons and negative ion for which get electron. The bond existing among these ions is known as an ionic bond. In today’s post, we will have a detailed look at these two bonds and compare them to find their differences and similarity. So let’s get started with the Difference Between Ionic and Covalent Bond.
Difference between Ionic and Covalent Bond
Ionic Bond
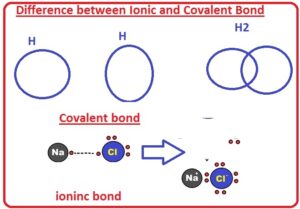
- The ionic bond is created among metallic and nonmetallic atoms. Nonmetals get electrons from metals to make bonds. The ionic bond is created due to the force of attraction between positive metallic ions and negative non-metallic ions.
- It is a chemical type of bond that has usage of electrostatic attraction among the ions.
- It has two types of ions structure the atom that gives make positive ion called cations
- The atom that gets electrons makes a negative ion called an anion.
- The electron transfer process called electrovalence
- Cation forms from metals and anion from non-metal.
- ionic element has lattice structure this structure defines through charges size
- ionic bond is caused due to redox reaction
- Na + Cl → Na+ + Cl− → NaCl
- The atom that has high electronegativity make a strong ionic bond
- At room temperature, it exists in the form of solids.
- Ionic compound generates current in solution form
- Their melting points have large according to ions arrangements.
- They can be soluble in water
- The larger the charge value there will be strong the force and the melting point of an ionic compound is high.
- The atoms created with the ionic bond do not have a specific shape.
- This bond also called the electrovalent bond created with the electrostatic attraction force among opposite charge ions in chemical compounds.
- There is a large melting point for atoms created with an ionic bond.
- Examples of atoms that have ionic bonds are sodium chloride.
- For the creation of this bond, there is a need for one metallic and one nonmetallic atom.
- The polarity of ionic bonds is high.
- In polar solvents such as water, ion compounds are soluble and make ions and current can flow these solutions.
Covalent Bond
- The covalent bond is created among 2 non-metallic atoms that have the same electronegativities. Both atoms share their electrons to make covalent bonds.
- It is created through sharing of electrons. The pair of electrons called bonding pairs.
- In organic chemistry, these bonds are commonly used
- There are different interaction occur in these bonds metal to metal, pi bonding, bent bonding.
- The atoms have a covalent bond have a certain shape.
- In hydrogen 2 electrons are shared.
- The atoms that have high electronegative have a high strength of the covalent bond.
- The sharing of electrons in more than two atoms called delocalized
- There are different types of covalent bonds sigma bond is the strongest bond creates due to the overlapping of orbitals
- The single bond is called a sigma bond
- A covalent bond is a fact through the electronegativity of atoms linked and defines chemical polarity.
- The atoms that have the same value electronegativity will have highlight nonpolar bond like hydrogen
- While different electronegativity has polar covalent bond HCL bond
- A pi bond is weak in nature create due to the overlap of p and d orbitals.
- The double covalent bond is created through the sigma and pi bond
- Their melting point is less than ionic bonds.
- This bond exists among 2 non-metals.
- Their boiling point is also less.
- At room temperature, it exists in the form of gases or liquid.
- The polarity of the covalent bond is low.
- The covalent compound is not soluble in water so current can not flow these solutions.
That is detailed post about Difference between Ionic and Covalent Bond if you have any query ask in comments. I tried my level best to make simple this post for. Thanks for reading. Soon I will upload next interesting article. Stay tune. have a good day.







