Hello, readers welcome to the new tutorial. we will discuss Difference Between Colorimeter and Spectrophotometer. these two are instruments that used for measuring the light-absorbing capacity of different materials. A spectrophotometer is used to measure the light transmitted and reflected when passes through any solution. While colorimeter is used to measure the quantity of solute in solution by measuring the light absorbed by the solution when passed to it.
In this post, we will discuss these meters working and other basic parameters to find differences. So let’s get started.
Difference Between Colorimeter and Spectrophotometer
What is Colorimeter
- It is a device that is used to find the value of light rays absorbed by the solution.
- It is used to find the quantity of solute existing in the solution through use of Beer-Lambert Law which says solute concentration is directly proportional to light absorbance.

Colorimeter Working
- The working principle of colorimeter is based on the Beer Ambert Law which states that light absorption and solute in the solution are directly proportional to each other.
- For measuring the color over the given standard colorimeter transit light through the liquid
- The lens of calorimeter and tristimulus absorption filter convert the light rays in single wavelength
- Photocell shows light absorbed and modules shows outputs the at the screen
- This meter is used for making a comparison between the new and old results.
- Its common uses are for measuring the yeast growth, asses beverage color and the quantity of ink used in printers.
- Its sensitivity is less and low cost
- It is a static meter
- It used for Psychophysical analysis and less sensitive
- Its cost is less and its structure is less complicated
What is Spectrophotometer
- The branch of electromagnetic spectroscopy that deals with quantitative measurements of reflecting features of material of light waves is called spectrophotometry.
- Spectrometry used photometers that are called spectrophotometers has the ability measure the intensity of light rays having different wavelengths
- Spectrometry is used for UV, visible and IR radiations but modern techniques are also applicable for electromagnetic spectrum like X-Ray microwaves
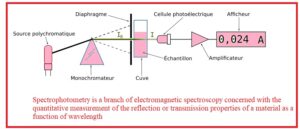
- It is device that used to measure the light absorbed by the colored compounds
- It main used for measuring the transmittance and reflection of solution of opaque and transparent objects.
- It used for physical analyses of substances
- Its sensitivity is larger than the colorimeter
- Expensive than the calorimeter
- Structure is more complicated then the calorimeter
- This meter is used in different since such as microbiology biochemistry physics etc
- Different particles used in drugs and medicines can be finds either they are helpful for use or not
- Used for measuring the bacterial growth and used for measuring the uric acid in urin
- For the progress of reaction, scientists used this meter
That is all about the . Difference Between Colorimeter and Spectrophotometer all details has been explained. If you have any further query ask in comments. Thanks for reading have a good day see you in next post







