 Hi, friends welcome to the new post. Here we will learn the Difference Between Anode and Cathode. Electrodes are the main element of any cell that is used to make a connection with other devices for the completion of circuits. There are two main categories of electrodes. The first one is the anode and the second one is the cathode.
Hi, friends welcome to the new post. Here we will learn the Difference Between Anode and Cathode. Electrodes are the main element of any cell that is used to make a connection with other devices for the completion of circuits. There are two main categories of electrodes. The first one is the anode and the second one is the cathode.
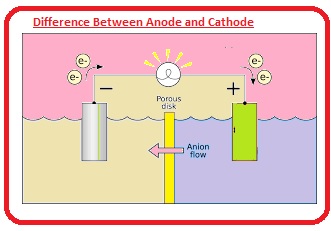
The anode is a positive terminal here oxidation process is done while the cathode is negative and reduction is done here. Here we will discuss the different parameters for these two modules in the detail. So let’s get started.
Difference Between Anode and Cathode
What is Anode
- The category of anode through which current goes to the different electrical components that have polar nature.
- The current passing in the diode has the nature of the conventional mean movement of positive charges.
- The oxidation process is done at the anode terminal example of this process is galvanic cells.
- The anode is also named as zincode.
- This terminal works as a donor since provides the electrons to the circuitry.
- In case of discharging of any module, the current is coming out from it.
- If a device is being charged then the current anode is working like the cathode.
- In primary types of cells there are no polarity changes in the anode and cathode while in the case of secondary cells polarity reversal occurs.
What is Cathode
- It is a category of the electrode that has a negative polarity and conventional current that goes out from it.
- Conventional current is current that is produced due to the movement of anion or positive charges.
- Due to negative polarity electrons move in the reverse direction of conventional current.
- In the case of the galvanic cells shows positive behavior.
- Consumption of charges is done at his point.
- it shows attraction for positive charges and repulsion for negative ones.
- This terminal is also named an acceptor of electrons.
- The reduction process is done at this module.
- In chemistry subject the point of the cell where reduction exists.
- It is denoted with the letter C.
- Electrons are given to the positive charge ions that move to the electrolyte.
- The movement of electrons from the cathode to parts of the solution is called cathode current.
Anode vs Cathode
- Anode and cathodes are two electrons employed in differnt types of electronic and electrochemical components. Here are some points discussed for comparison.:
- Charge: The cathode is negatively charged and the anode has a positive charge.
- Electron flow direction: The direction is anode to the cathode for electrochemical cells while for diodes and other electronic devices the direction of current from anode to cathode.
- Chemical Reaction: Oxidation take place at the anode and loss of electrons occurs while at the cathode electrons gain and a reduction reaction occurs.
- Position in a cell: In the case of a galvanic cell the electrode where oxidation occurs is called the anode and exists on the left side. Cathode has a reduction reaction and exists at the right side
- Polarity: The anode is attached to a positive point of the battery or other power sources and the cathode exists at the negative terminal of the battery.
- Functions: Electrons are produced at the anode and cathodes get the electrons
What is a positive electrode?
- The positive electrode is a type of electrode that has a positive charge. It is considered as the site of reduction reaction since positive charges or cations are moved to the electrode and get electrons and make neutral atoms or molecules.
- In the case of a cell or battery positive electrodes are called the cathode and it is attached to the positive terminal of the power source.
- Positive electrodes are used in different applications such as batteries, electrolysis cells, and fuel cell, in these applications they help to generate and store electrical energy
What is a Negative Electrode
- It is the type of electrode that is a negative charge in an electrochemical system. At this point oxidation reaction take place and negative ions or anions are attracted and lose an electron to become neutral atoms or molecules. Negative electrodes in the battery or cell are called anodes and are attached to the negative point of the power source. These electrodes are used in electrolysis cells, fuel cells, and batteries like cathode or positive electrodes to generate and store energy.
FAQs:
- Is A cathode positive or negative?
- The cathode is a negative electrode
- What is an anode and cathode example?
- An example of an anode and cathode is a battery. The anode is created with the use of reactive metallic material like zinc and inert metals used for the creation of a cathode such as copper. During their working anode faces oxidation and electrons emit that move to the cathode through an external circuitry and a reduction reaction occurs at the cathode through the gain of electrons
Does oxidation occur at the anode or cathode?
- Oxidation takes place at the anode
What are the materials used for the anode and cathode?
- The material used for anode and cathode is on the basis of their applications. For instance, in the case of battery anode is created with lithium or zinc, and the cathode is created with cobalt oxide or manganese oxide
Why is cathode positive?
The cathode is negative, not positive. The cathode work as the site where the reduction process takes place, where it receives electrons and becomes negatively charged, which may cause confusion in some applications, such as electrolysis cells, where the roles of the anode and cathode are reversed.
Is the anode positive or negative? Explain in details
Although it is the location of an oxidation reaction, where it loses electrons and becomes positively charged, the anode is normally positive in terms of its charge in an electrochemical system. The cathode, which is connected with a positive point of the cell or power source gets electrons while the anode generates electrons that are connected with the negative terminal of power source electrons.
is cathode positive or negative? Explain in details
A cathode is negative in form of charge in the electrochemical cell. through it is a point where a reduction reaction takes place and electrons are gained and become negatively charged. In a battery or cell, the cathode is attached to the positive point and it gets electrons, whereas the anode is attached to a negative point and releases electrons
Why is cathode positive?
The cathode is negative, not positive. The anode and cathode’s functions are inverted in some applications, such as electrolysis cells, where the cathode acts as the location of a reduction process, where it picks up electrons and acquires a negative charge.
Why is the cathode negative?
The cathode is negatively charged because it undergoes a reduction process there, where it picks up electrons and changes its charge. Anode has attached to the negative terminal its electrons are releases while the cathode is attached to the positive point and its electrons are taken
which of the following best differentiates a positive electrode from a negative electrode?
The basic difference between positive and negative electrodes is the direction of electron flow. In an electrochemical system positive electrode is a position where reduction takes place there electrons losses become a positive charge
when testing electrodes for polarity, the negative electrode will create:
When electrodes are tested for polarity at negative electrodes oxidation reaction takes place their electrons lose and transformed into positively charged
That is a detailed post about the Difference Between Anode and Cathode. If you have any further queries ask in the comments. Thanks for reading have a good day.